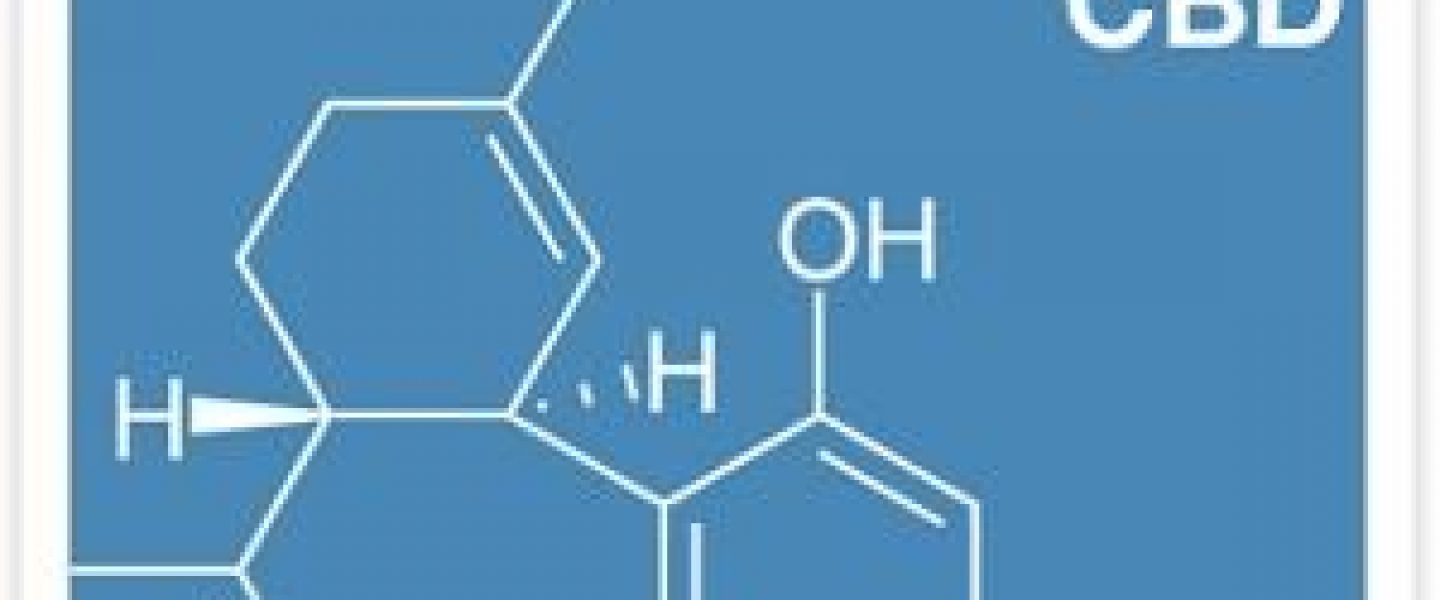
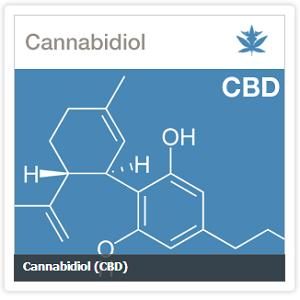
 Yesterday, the Senate International Narcotics Control Caucus held a hearing on facilitating cannabidiol (CBD) research. CBD is found in cannabis and has shown great potential to treat intractable epilepsy and numerous other therapeutic benefits. According to the Federal Drug Administration (FDA) CBD is considered a schedule I substance, despite the growing body of evidence indicating therapeutic benefits to patients suffering from dravet syndrome and other severe forms of epilepsy. The hearing also focused on regulatory oversight and reforming the process for researchers to obtain cannabis.
Yesterday, the Senate International Narcotics Control Caucus held a hearing on facilitating cannabidiol (CBD) research. CBD is found in cannabis and has shown great potential to treat intractable epilepsy and numerous other therapeutic benefits. According to the Federal Drug Administration (FDA) CBD is considered a schedule I substance, despite the growing body of evidence indicating therapeutic benefits to patients suffering from dravet syndrome and other severe forms of epilepsy. The hearing also focused on regulatory oversight and reforming the process for researchers to obtain cannabis.
“During today’s hearing we heard Nora Volkow, the head of National Institute on Drug Abuse (NIDA), acknowledge that researchers would benefit from ending the monopoly that NIDA holds on producing cannabis for research,” said Christopher W. Brown ASA Press Secretary. “The DEA-mandated NIDA monopoly on producing cannabis is slowing down research that everyone agrees needs to move forward quickly; it’s long past the time we let other professional growers produce cannabis for researchers.”
Earlier this week the Office of National Drug Control Policy (ONDCP) announced they will lift the Public Health Service review on marijuana research projects not funded by the federal government in a move researchers hailed as lifting a burdensome and unnecessary barrier to cannabis research. However, the NIDA monopoly on producing research cannabis has remained in place.
“Marijuana is the only schedule one drug that is forced to deal with this single government enforced monopoly for access to study drug, said Dr. Sue Sisley. ” This monopoly has proven over and over again that they are incompetent and incapable of providing the various strains that are requested by scientists in a timely manner.”
In 2014, Dr. Sisley received approval from the FDA for a major study on cannabis use to treat post-traumatic stress disorder, however the study continues to be on hold as NIDA has not been able to produce the requested strains of cannabis.
Further Information:
Ending NIDA’s Monopoly ASA Backgrounder
Office of National Drug Control Policy Lifts Public Health Service Review on Marijuana Research
Source: Americans for Safe Access – make a donation